Batteries
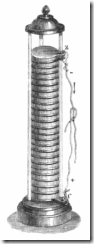 A battery consists of one or more cells, each of which use stored chemical energy to produce electrical energy, There are many types of cells and these are combined in a multitude of ways to produce the many battery types in use.
A battery consists of one or more cells, each of which use stored chemical energy to produce electrical energy, There are many types of cells and these are combined in a multitude of ways to produce the many battery types in use.
Batteries are divided in to primary and secondary:
- Primary batteries – have an irreversible chemical action and can only be used once
- Secondary batteries – have a reversible chemical action and can be recharged
The modern battery came into being due mainly to work carried out by Alessandro Volta at the end of the 18th century. He was able to make a cell by using silver and zinc discs sandwiched between cardboard soaked in salt water. By combining many cells in series he created what has become known as the Voltaic Pile.
Theory
When two dissimilar metals [electrodes] are placed in an electrolyte, ions will flow from the more reactive electrode to the other, building up charge. This build up of charge is neutralised by the flow of electrons [current] in the circuit.
Splug.com (physics) have a good article which looks at the chemistry of how a cell works in more detail. A link to the page is included below.
Approximate EMFs of Cells
| Cell | V |
| Bichromate | 2 |
| Bunsen | 1.9 |
| Dry cell | 1.5 |
| Daniell | 1.08 |
| Grove | 1.8 |
| Lead-Acid | 1.85 - 2.2 |
| Leclanché | 1.46 |
| Lithium Copper Oxide | 1.7 |
| Lithium Iron Disulfide | 1.5 |
| Lithium Manganese Dioxide | 3.0 |
| Lithium Ion | 3.6 |
| Mercury Oxide | 1.35 |
| Nickel Cadmium | 1.3 |
| Nickel Iron | 1.4 |
| Nickel Metal Hydride | 1.2 |
| Nickel Oxyhydroxide | 1.7 |
| Nickel Zinc | 1.6 |
| Silver Oxide | 1.55 |
| Zinc Air | 1.35 – 1.65 |
| Zinc Carbon | 1.5 |
| Zinc Chloride | 1.5 |
| Zinc Silver Oxide | 1.8 |
Capacity (Ampere-hour)
Capacity measures the amount of energy stored in a battery. In a battery, capacity is measured in ampere-hours (Ah). This is the amount of amperes which can be drawn from the battery for one hour. If a lower current is drawn the battery will last longer than an hour (conversely if more current is drawn it will last less).
In practice, varying terminal voltages, temperature and other factors make the assessment of capacity complex.
Internal resistance
Batteries are not perfect devices. To understand their operation they can be modelled as a perfect voltage source (one with zero internal resistance) in series with an internal resistance. The voltage Vt at the terminals of a battery is given by:
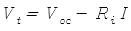
- Voc is the open-circuit voltage of the battery
- Ri is the battery's internal resistance
- I is the current flowing through the battery
This can be rearranged to calculate the internal resistance given the other quantities:
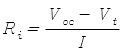
As the battery runs down and its internal resistance increases. Consequently the voltage drop across the internal resistance increases and the terminal voltage decreases.
Battery Types
Alkaline Battery - invented by Canadian engineer Lewis Urry in the 1950s, alkaline batteries are a type of primary battery or rechargeable battery which utilizes alkaline as the electrolyte . Alkaline batteries have a higher output than many other battery types and account for the largest share to the battery market worldwide.
Common Consumer Battery Sizes
There are a vast number of battery types in use. The table below lists the most common, which are used in everyday appliances. For a more complete list, Wikipedia has a good page – see the external links section below.
| Diagram | Size | Capacity
(mAh) | V | ANSI
NEDA | IEC | Diam.
(mm) | Height
(mm) | Mass
(g) |
| | AAAA | 625 | 1.5 | 25A | LR8D425 | 8.3 | 42.5 | 6.5 |
 | N | 1000 | 1.5 | 910A | LR1 | 12 | 30.2 | 9 |
 | AAA | 1250 | 1.5 | 24A | LR03 | 10.5 | 44.5 | 11.5 |
 | AA | 2850 | 1.5 | 15A | LR6 | 14.5 | 50.5 | 23 |
| | J | 625 | 6 | 1412A | 4LR61 | prismatic | 48.5 | 30 |
| | 9V | 625 | 9 | 1604A | 6LR61 | prismatic | 48.5 | 45.6 |
 | C | 8350 | 1.5 | 14A | LR14 | 26.2 | 50 | 66.2 |
 | D | 20500 | 1.5 | 13A | LR20 | 34.2 | 61.5 | 148 |
Notes: capacities are typical only.
Battery Life
Primary Batteries – naturally lose 8% to 20% per year at a temperature of about 20°-30°C due to self discharge. Lowering the battery temperature (keeping in the fridge for example, can aid in reducing the self discharge.
Secondary Batteries – generally self-discharge quicker than primary batteries. As batteries are recharged, they lose capacity – typical recharge cycles are in the range of 500 to 1000 charges.
Battery life can be seriously affected by temperature. The link [effect of temperature on lead batteries], at the end of the article, explains this phenomena in detail.
External Links